AS4187 Compliance is tricky and takes a good understanding of the requirements of the standard and a very good understanding of your system performance.
In order to meet the requirements of AS4187 there is some serious data analysis required. This includes a carefully considered sampling programme to measure current performance to understand the existing performance and capacity of existing systems.
View data in a simple real-time page to understand key performance indicators.
Developing this monitoring program is either done in house in collaboration with the CSSD Manager and infection control team or external consultants like Ecosafe International who have technically qualified consultants who have assisted many clients along this journey.
When it comes to sampling, results management, data visibility and display of performance, this is where Do Diligence work with many health care clients to provide a clear picture of performance and the key areas of focus.
Using real-time data, our clients can view the performance of the systems and understand how the systems are trending and when early preventative action is required to ensure that systems remain operational and within safe parameters for the facility.
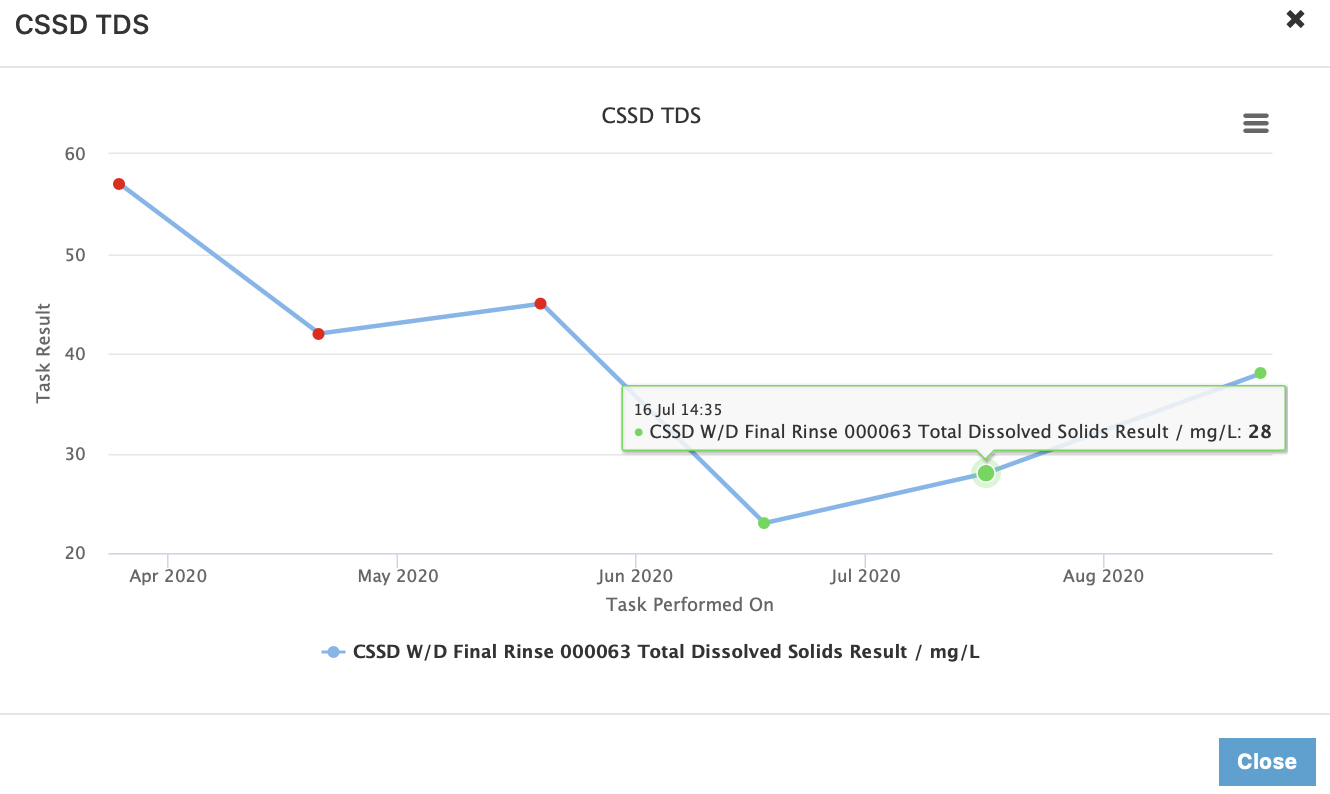
Some of our clients are too busy to login to the real-time interactive dashboard and so for those clients we provide auto reports. The auto report provides a management overview and / or exception reporting. This means that CSSD Managers, Infection & Prevention Control Managers and Clinical Managers receive only key information to have a good understanding of performance and (if by exception) their attention is required.
Ease of use and visibility of data is critical to moving to and remaining compliant with the AS4187 standards.
As many of our clients appreciate, not only is achieving compliance with AS4187 critical, but maintaining ongoing compliance and demonstrating compliance is a key focus for all CSSD departments. Our compliance software is used across the healthcare industry to ensure not only are the key samples taken, but also that the key operational tasks are completed and measured. This operational data demonstrates compliance to the management plan and is useful for the providing key information during audits or reporting to management.
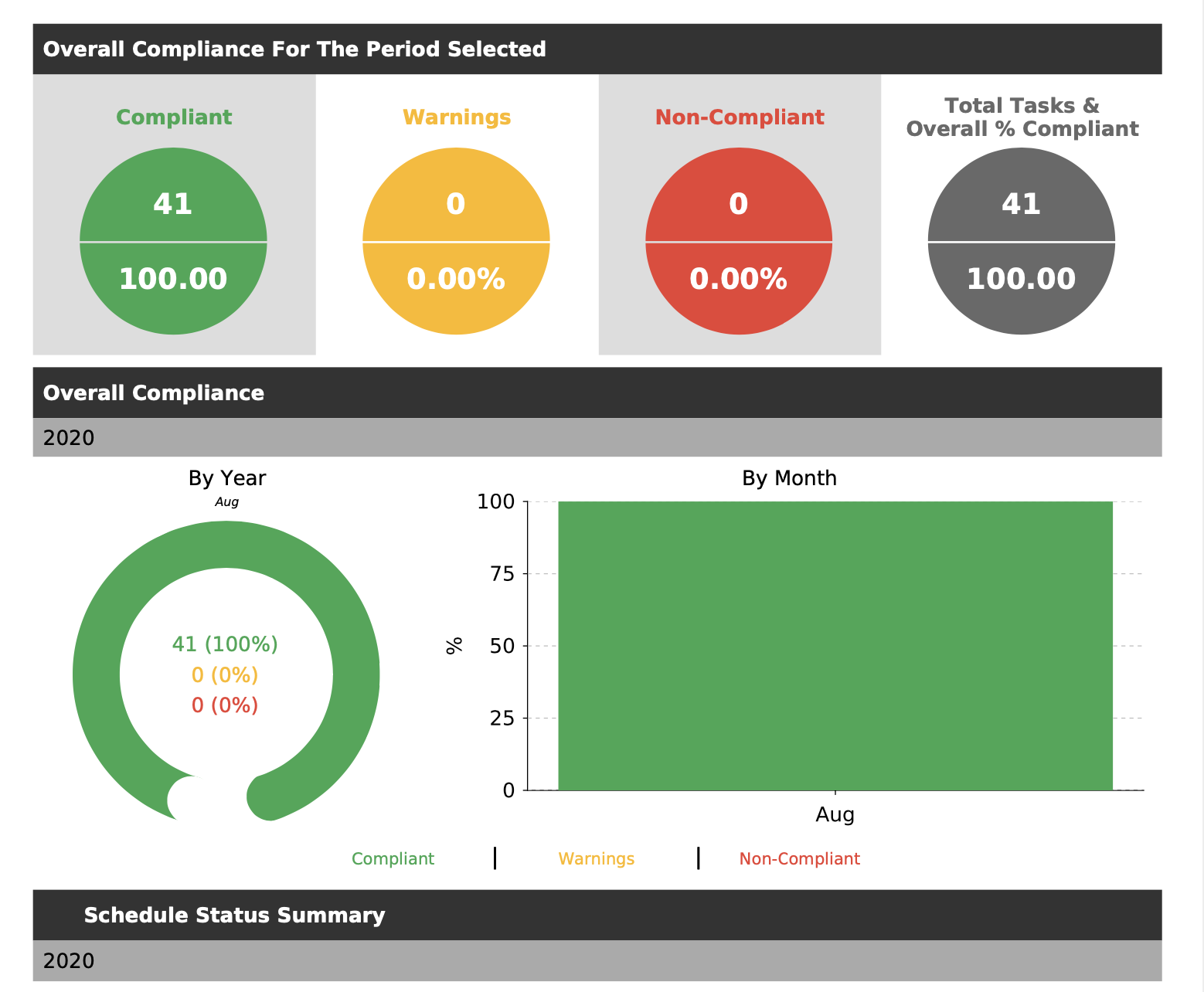
A key facility that Do Diligence and our partners offer is an electronic data processing system. This means there are no human errors from the chain of custody to the results return and upload. All of these tasks are completed electronically which not only reduces the possibility of data errors to almost zero, but also means all results are processed in seconds with no laborious data entry, leaving the clients staff focus on the day to day tasks of managing the CSSD facility.
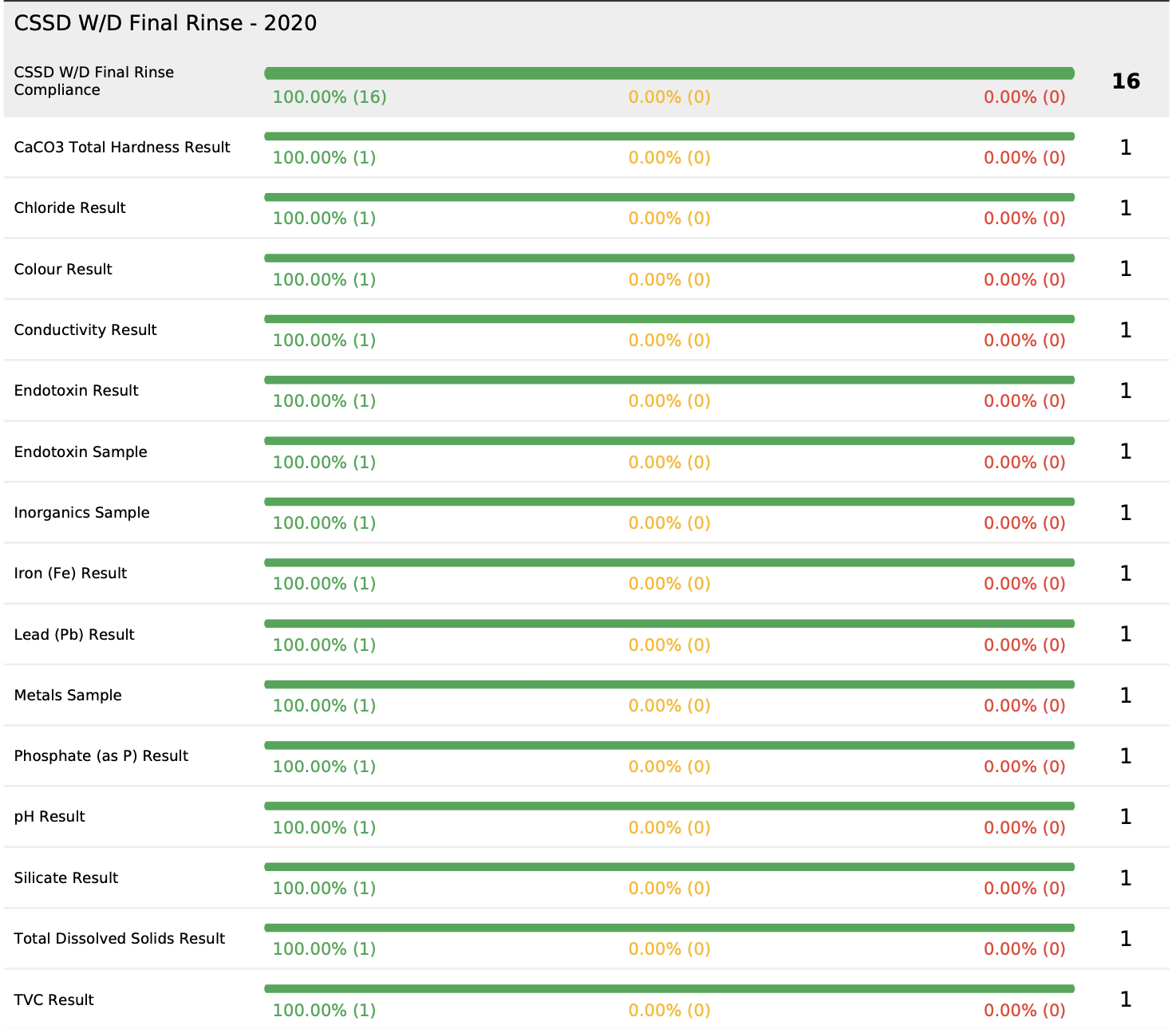
If you would like to understand further how we are currently working with some world class facilities in the Australian Health Care Space, please get in touch and we can provide an overview